Second Law of Thermodynamics
- Admin

- Aug 28, 2020
- 2 min read
It may be defined by two statements
1. Kelvin Plank’s statement
2. Clausius statement
1. Kelvin Plank’s statement
Kelvin Plank’s statement deals with the heat engines. According to this statement of 2nd law, it is impossible to construct any engine working in a cyclic process which has no effect other than receiving heat from high temperature source and converting it into equal amount of work.
In other words it is impossible to any heat engine working in a cyclic process whose sole effect is to receive heat from high temperature source and convert the same amount into equal amount of work.
From this definition for any heat engine to be feasible some amount of heat must be rejected to low temperature sink, while producing work output
Let us consider a cyclic heat engine which is working in a cyclic process.
We know that

Ƞ = desired effect ----(1)
input
Desired effect = Wₜ – Wₚ
As the engine is working in a cycle
ƩQ = ƩW { from 1st Law of Thermodynamics}
⇒ Q₁ - Q₂ = Wₜ – Wₚ
Ƞ = Wₜ – Wₚ
Q₁
= Q₁ - Q₂
Q₁
= 1 - Q₂
Q₁
= 1 - Q rejected
Q supplied
Now if Q rejected = 0
Then Ƞ heat engine = 1-(0/Q supplied) = 1 or 100%
From this definition of 2nd Law of Thermodynamics, heat rejected can never be zero. Therefore efficiency of heat engine is always less than 100%.
A hypothetical device which violate the Kelvin Plank’s statement and given 100% efficiency by producing work output without rejected any heat to the sink is called Perpetual Motion Machine of 2nd kind (PMM-2).
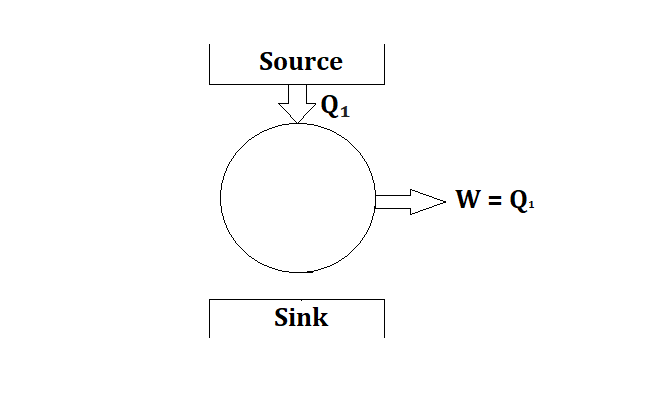
2. Clausius Statement
According to this statement, it is impossible to construct any device whose sole purpose is to take heat from low temperature body and transfer this heat to high temperature body without the help of any external work.

Any device which takes heat from low temperature body and reject the same heat to high body without the help of any external work is also called Perpetual Motion Machine of 2nd kind (PMM-2).
From this statement of 2nd Law of Thermodynamics, we can say that for any device to take heat from low temperature and reject heat to high temperature some work input must be required.


Refrigerator and Heat Pump works on the Clausius Statement. Although the cycle of operation of both these devices is same but there functions are different. Efficiency of these reverse Heat Engines is describe by term COP ( Coefficient of Performance).

Comments